Deviation from Ideal Behaviour
Deviation from Ideal Behaviour: Overview
This Topic covers sub-topics such as Difference between Ideal Gases and Real Gases, Compressibility Factor, Real Gas, Deviations from Ideal Gas Behaviour, Compressibility of Gas and, Variation of Compressibility Factor with Pressure
Important Questions on Deviation from Ideal Behaviour
A gas is said to behave like an ideal gas when relation constant. When do you expect real gas to behave like an ideal gas?
Van der Waal’s real gas, act as an ideal gas, at which conditions ?
At which one of the following temperature – pressure conditions the deviation of a gas from ideal behaviour is expected to be minimum ?
A gas has a compressibility factor of and a molar volume of at a temperature of and pressure . If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be . The value of is _________
[Use: Gas constant, ]
A certain quantity of real gas occupies a volume of at and when its compressibility factor is Its volume at and (When its compressibility factor is ) is (Nearest integer)
A gas at and has molar volume percent smaller than that for an ideal gas under the same conditions. The correct option about the gas and its compressibility factor is :
For which of the following gases, the value of Z is greater than 1 at all pressures and at 273 K?
Following data is given for
Calculate the mass (g) of required to fill 500 mL flask under these conditions.
Consider the following statements :
I. Density and molar mass of gaseous substance are related as
II. Partial pressure in terms of molefraction is related as
III. At constant temperature, plot for real gases is a straight line
IV. Real gases do not follow ideal gas equation perfectly under all conditions
Choose the correct statements and select the correct option
Gases deviate from ideal behaviour at high pressures because the gas molecules
At and bar pressure, the measured volume of mole of a gas is . The van der Waals constant and compressibility factor , respectively are
Among the gases and , the gases that show only positive deviation from ideal behavior at all pressures in the graph are
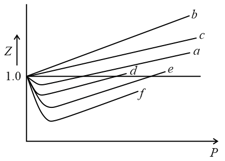
Ideal gas is an imaginary gas but real gas deviate from ideal gas behaviour. How is it possible?
In the following isotherms, (for ideal gas) and II (for real gas), points and on with respect to I indicate _____ deviation
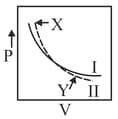
Select the incorrect statement(s):
Gases are compressible because
Why gases are compressible?
The internal energy per mole of an ideal gas is denoted by . The same for a real gas obeying van der Waals equation is . Assuming the average kinetic energy of the molecules of the two gases at the same temperature to be equal, the correct statement for the gas at low pressure is
Regarding gas, the correct statement(s) is/are
The correct statement(s) regarding compressibility factor is/are
